Abstract
Background
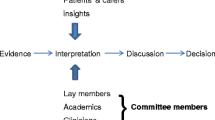
The National Institute for Health and Care Excellence (NICE) produces guidance on the use of health technologies (including new and existing medicines, medical devices, diagnostics and interventional procedures) in the National Health Service. Technology Appraisals inform recommendations on the use of new and existing health technologies. As part of its health technology evaluation process, NICE ask independent research groups known as Evidence or External Assessment Groups (EAGs) to assess or evaluate the available evidence surrounding health technologies. Although patients and the public are involved in the wider NICE Heath Technology Evaluation and Assessment process, little is known about the extent to which patient and public involvement and engagement (PPIE) is undertaken and documented in EAG Reports.
Objectives
This rapid scoping review aimed to discover the extent to which PPIE is currently undertaken and documented in EAG Reports, which feed into the wider NICE health technology assessment process, and whether EAG Reports contain a plain language summary.
Methods
We searched the NICE website for guidance published between 27 September, 2022 and 27 September, 2023. All records were downloaded directly from the NICE website into an Excel spreadsheet for extraction. Evaluations that were terminated before guidance was published or where an EAG Report was not available as supporting evidence were excluded. One researcher charted information regarding the type of each EAG Report, whether a plain language summary was included, and whether documentation of PPIE was included in the EAG Report either within a stand-alone section or throughout the main text of the report. A second researcher checked charted information for 20% of these records. We tabulated data and described PPIE conduct and documentation in included EAG Reports within a narrative synthesis.
Results
A total of 97 EAG Reports were included in this rapid scoping review, the majority of which were documenting Single Technology Appraisals (N = 55). Of the 97 EAG Reports, 11 included a plain language summary. Of these 11 reports, two were Multiple Technology Appraisals, five were Diagnostic Assessment Reviews and four were Early Value Assessments. One Early Value Assessment, one Diagnostic Assessment Review and one Multiple Technology Appraisal reported that they did not conduct PPIE because of time constraints and noted that patients were involved in the wider NICE Appraisal process. Two Early Value Assessments that explicitly reported on PPIE used heterogenous methods of involvement.
Conclusions
There is currently limited PPIE documented in EAG Reports and inclusion of a plain language summary is uncommon. Further guidance is required to assist EAGs with embedding PPIE and a plain language summary into their Reports taking into consideration the ultra-rapid nature of the production of these reports.
Plain Language Summary
In the UK, the National Institute for Health and Care Excellence produces guidance on what medicines, medical devices and diagnostic tests can be used within the National Health Service. The National Institute for Health and Care Excellence ask groups of independent researchers to look at information about these health technologies (including how well they work, how safe they are and how much they cost) and produce a Report to help them decide on whether they should be used in the National Health Service. Although patients and the public are involved in other stages of this process, we do not know how much patients and the public are involved in the Reports that these independent research groups produce. We wanted to find out how often patients and the public were included in these Reports. We also wanted to find out how many of these Reports contained a plain language summary, which is a useful tool in helping members of the public understand research. To do this, we looked for independent research group Reports that had been published between September 2022 and September 2023. We then read them to see if they involved patients or members of the public, or if they included a plain language summary. We included 97 Reports in our work. Of these 97 Reports, only two involved patients and the public and 11 contained a plain language summary. Based on what we found, we believe there needs to be further guidance on how to involve patients and the public, as well as how to write plain language summaries for these independent Reports.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
National Institute for Health and Care Excellence. Technology appraisal guidance. 2023. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance. Accessed 21 Oct 2024.
National Institute for Health and Care Excellence. Technology appraisal guidance. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance. Accessed 21 Oct 2024.
National Institute for Health and Care Research. Briefing notes for researchers: public involvement in NHS, health and social care research. April 2021. https://www.nihr.ac.uk/documents/briefing-notes-for-researchers-public-involvement-in-nhs-health-and-social-care-research/27371. Accessed 21 Oct 2024.
National Institute for Health and Care Excellence. Involvement and participation. https://www.nice.org.uk/process/pmg36/chapter/involvement-and-participation. Accessed 21 Oct 2024.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Westwood M, Armstrong N, Krijkamp E, Perry M, Noake C, Tsiachristas A, Corro-Ramos I. CaRi-Heart® for predicting cardiac risk in suspected coronary heart disease (CAD): a rapid review and conceptual economic model to inform Early Value Assessment (EVA). Kleijnen Systematic Reviews Ltd; 2022.
Harman S, Navega Biz A, Hamilton J, Whyte S, Simpson E, Ren K, et al. Quantitative faecal immunochemical tests to guide colorectal cancer pathway referral in primary care. 2023. Available at: https://www.nice.org.uk/guidance/dg56/history.
Metry A, Pandor A, Ren S, Shippam A, Clowes M, Dark P, et al. Therapeutics for people with COVID-19: an economic evaluation. School of Health and Related Research (ScHARR); 2022.
Tomlinson E, Ward M, Cooper C, James R, Stokes C, Begum S, et al. Point of care tests for urinary tract infections (UTI) to reduce antimicrobial resistance: a systematic review and conceptual economic model to inform Early Value Assessment (EVA) (DAP 69). Bristol Technology Assessment Group; 2023.
Shabaninejad H, Kenny RPW, Robinson T, Stoniute A, O'Keefe H, Still M, et al. Early Value Assessment: Genedrive MT-RNR1 ID Kit for detecting single nucleotide polymorphism m.1555A>G in neonates. 2022. Available at: https://www.nice.org.uk/guidance/hte6/history.
Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Development of the ACTIVE framework to describe stakeholder involvement in systematic reviews. J Health Serv Res Policy. 2019;24(4):245–55.
Garrity C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C, et al. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13–22.
Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358: j3453.
National Institute for Health and Care Research. Plain language summaries. 2021. https://www.nihr.ac.uk/documents/plain-english-summaries/27363. Accessed 5 Apr 2021.
Cochrane. Updated template and guidance for writing plain language summaries in Cochrane Reviews now available. 2022. https://community.cochrane.org/news/updated-template-and-guidance-writing-plain-language-summaries-cochrane-reviews-now-available. Accessed 21 Mar 2023.
Acknowledgements
We thank Debbie Smith for commenting on the manuscript and reviewing the plain language summary.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Eugenie Evelynne Johnson was supported by the Newcastle University Research Excellence Development Fund.
Conflict of interest
Eugenie Evelynne Johnson and Cyril Onwuelazu Uteh work as Clinical Effectiveness Reviewers for the Technology Appraisal Group based at Newcastle University, UK. Emma Belilios works within the External Assessment Group based at Newcastle upon Tyne Hospitals NHS Foundation Trust, UK. Fiona Pearson is a co-applicant for the Technology Appraisal Group at Newcastle University, UK and works as a Clinical Effectiveness Reviewer for both this Group and the External Assessment Group based at Newcastle upon Tyne Hospitals NHS Foundation Trust, UK. The authors did not receive support from any organisation for the submitted work.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
The authors performed the following tasks, in accordance with the CRediT taxonomy: EEJ: conceptualisation, methodology, data curation, investigation, formal analysis, writing (original draft), project administration; COU: data curation, investigation, validation, writing (review and editing); EB: writing (review and editing), supervision; FP: writing (review and editing), supervision.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Johnson, E.E., Uteh, C.O., Belilios, E. et al. Reporting of Patient and Public Involvement in Technology Appraisal and Assessment Reports: A Rapid Scoping Review. Patient 18, 109–114 (2025). https://doi.org/10.1007/s40271-024-00721-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-024-00721-7